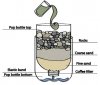
to make water drinkable, if from an unknown source, then the water must be processed in a number of ways
1) larger particulate mater must be removed - this can be done simply by passing the water through a fine mesh ( a T-shirt, or if available fine sand)
2) Larger protozoa needs to be killed- this can be achieved in one of two ways a) chemical treatment of the water using something like aqua pure tablets or boiling for about 10 mins (personally I prefer boiling, as recent research has shown that some protozoa are quite resistant to the chemicals (Uni of Glasgow study of water borne threats for the world health organisation)
3) removal of chemical and heavy metals- This is more difficult as it requires something like activated charcoal with a fine mesh (20x50) in emergencies you can use charcoal from a fire However this is only going to be partially effective
Water has a lower boiling temperature that the more harmful Metals (Arsenic, Benzine,Lead, potassium) but is less than some compounds (Ethanol, petrochemicals etc)
however boiling water and allowing these lower temperature compounds to escape before condensing you stream back into water will remove most of these.
The general view really is that if you are in a situation where you are treating possible harmful water then, your body can sustain small amounts of these metals over short periods, BUT a better source should always be explored
Hope this helps
Day